Abstract
Introduction: Nucleophosmin 1 (NPM1) mutations without an FMS-like tyrosine kinase-3 ITD (ITD) mutation are associated with a favorable prognosis in younger (age <60 years) patients (pts) with acute myeloid leukemia (AML). However, the prognostic impact of NPM1 mutations with concurrent low ITD allelic ratio (ITDlow) is unclear. Furthermore, the benefits of hematopoietic stem cell transplant (SCT) in NPM1+ AML in pts >60 is not known. In this retrospective study, we investigated the impact of FLT3 mutational status in pts with NPM1+ AML and the role of SCT in patients >60 years with ITD negative (ITDneg).
Methods: We included patients from January 2013 to August 19, 2021 from 7 centers with NPM1+ AML who received induction chemotherapy with an anthracycline and cytarabine. Data was collected by chart review and mutational status was determined by next generation sequencing at each institution according to local guidelines. ITDhigh was defined as an allelic ratio (AR) of ≥0.5; AR <0.5 was considered ITDlow. The log-rank test was used to compare overall survival between groups.
Results: A total of 345 pts met the inclusion criteria. The mean age was 58 years (range 18-79) and 56% were female. Normal karyotype was seen in 80%. ITD was detected in 142 of the 345 (41%); a FLT3-TKD mutation was detected in 48 (14%) pts. Common co-mutations included DNMT3A (42%), TET2 (23%), IDH2 (19%), IDH1 (19%), and RAS (19%). The complete response (CR) rates for NPM1+/FLT3neg pts was 79.8%; the CR rates for the NPM1/ITDpos pts treated with IC+TKI, IC alone, and NPM1+/ITDneg/TKD+ were 73.3%, 74.1%, and 80%, respectively.
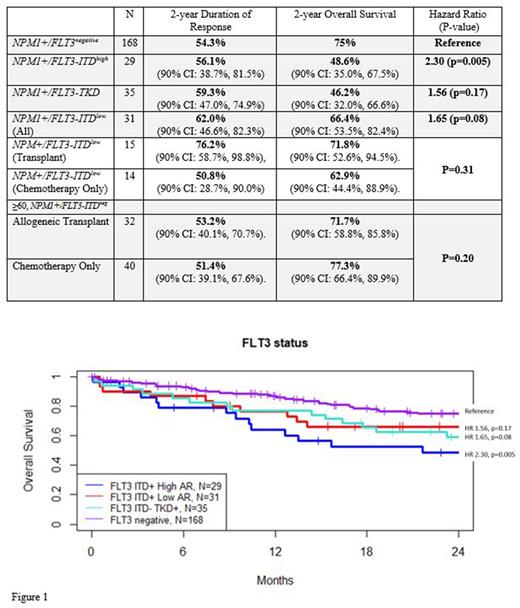
In the entire ITD+ population, AR was known in 42.3% (N=60) of pts. The 2-year overall survival (OS) for the NPM1+/ITDhigh (N=29) was 48.6% (90% CI: 35.0%, 67.5%) and the 2-year duration of response (DOR) was 56.1% (90% CI: 38.7%, 81.5%), while the NPM1+/ITDlow group (N=31) had a 2-year OS of 66.4% (90% CI: 53.5%, 82.4%) and 2-year DOR of 62.0% (90% CI: 46.6%, 82.3%). The comparison of OS in these two groups by log-rank was not significant (p=0.31). In the ITDlow group, pts who had SCT (N=15) had a 2-year OS of 71.8% (90% CI: 52.6%, 94.5%) and a 2-year DOR of 76.2% (90% CI: 58.7%, 98.8%); those treated with chemotherapy alone (N=14) had a 2-year OS of 62.9% (90% CI: 44.4%, 88.9%) and 2-year DOR of 50.8% (90% CI: 28.7%, 90.0%). OS was not significantly different (p=0.35). Comparing survival between FLT3 groups with a Cox PH model, FLT3-ITDhigh was significantly associated with inferior OS vs. FLT3neg(HR = 2.30, p=0.005). However, FLT3-ITDlow and FLT3-TKD+ were not associated with inferior OS vs. FLT3neg (HR=1.56, p=0.17 and HR=1.65, p=0.08, respectively).
In pts >60 and NPM1+/ITDneg (N=104), the mean age was 67 years (range 60-78), median WBC count at baseline 7.7 (range 0-127), and 48.1% of pts were female. The CR rate was 75.0% (N=78). A total of 32 pts underwent SCT and 40 pts received a median of 3 cycles of consolidation. The 2-year OS and DOR in the SCT group was 71.1% (90% CI: 58.8%, 85.8%) and 53.2% (90% CI: 40.1%, 70.7%), respectively as compared to 77.3% (90% CI: 66.4%, 89.9%) and 51.4% (90% CI: 39.1%, 67.6%), respectively in the non-SCT group. Albeit with small numbers SCT did not improve OS in this age group (p=0.2)
In the entire cohort, the five most common co-mutations other than FLT3 were evaluated for influence on survival. Using the log-rank test, only DNMT3A and TET2 were associated with inferior OS (P=0.026 and 0.039, respectively), while IDH1, IDH2, and RAS were not significantly associated with survival (p-values of 0.43, 0.6, and 0.69, respectively).
Conclusion: In our multicenter cohort of pts with NPM1+ AML, OS was significantly inferior in pts with ITDhigh v. FLT3neg, but not in ITDlow and TKD. Co-mutations in DNMT3A and TET2 adversely affected overall survival. Our data did not find a significant difference in OS in pts >60 with NPM1+/FLT3-ITDneg who were consolidated with chemotherapy versus SCT, though larger follow up studies are needed.
Disclosures
Winer:Curis: Consultancy; Novartis, Jazz Pharmaceuticals, Pfizer, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Takeda Pharmaceuticals: Consultancy. Shallis:Bristol Myers Squibb and Gilead Sciences, Inc: Honoraria; Gilead Sciences, Inc.: Honoraria. Goldberg:DAVA Oncology: Honoraria; Moderna, Novavax: Current equity holder in publicly-traded company; Genentech: Consultancy; Aprea, Aptose, AROG, Celularity, Pfizer, Prelude Therapeutics: Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Stahl:Curis Oncology: Consultancy; Boston Consulting: Consultancy; Novartis: Other: Advisory Board. Patel:AbbVie: Consultancy; Celgene/BMS: Research Funding; Servier/Agios: Research Funding; Pfizer: Research Funding; Kronos Bio: Research Funding. Duvall:Jazz Pharmaceuticals: Consultancy. Abaza:Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; ALX Oncology: Research Funding. Luskin:Pfizer: Honoraria; Abbvie: Research Funding; Novartis: Research Funding. Stone:BMS: Consultancy; Jazz: Consultancy; Astellas: Consultancy; Elevate Bio: Consultancy; Abbvie: Consultancy, Research Funding; Aprea: Consultancy; Actinium: Consultancy; GSK: Consultancy; Kura Oncology: Consultancy; Takeda: Consultancy; Janssen: Consultancy; Syndax: Consultancy; Syntrix: Consultancy; Syros: Consultancy; Apteva: Consultancy; Arog: Consultancy, Research Funding; Novartis: Consultancy; Boston Pharmaceuticals: Consultancy; BerGenBio: Consultancy; Innate: Consultancy; Epizyme: Consultancy; Foghorn Therapeutics: Consultancy; Gemoab: Consultancy; OncoNova: Consultancy. DeAngelo:AbbVie, Blueprint Medicines Corporation, GlycoMimetics, and Novartis: Research Funding; AbbVie, Amgen, Autolus, Blueprint Medicines Corporation, Forty-Seven, GlycoMimetics, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal